Pharmaceuticals
Ingestible & Topical Applications

Koster Keunen Wax Excipients
For over 170 years, Koster Keunen has been a global leader in wax manufacturing and innovation. Founded in 1852, Koster Keunen has pioneered advancements in wax technology, earning a reputation for unmatched quality, consistency, and technical expertise. With multiple manufacturing facilities worldwide and the unique capability to support internal Disaster Recovery Planning (DRP), Koster Keunen remains the trusted partner for pharmaceutical and topical applications, delivering solutions that meet the most stringent industry standards.
In pharmaceuticals, wax excipients play a vital role in ensuring stability, functionality, and aesthetic quality of formulations. Whether in controlled-release drug delivery systems, tablet coatings, or topical emulsions, waxes provide essential benefits that enhance product performance and consumer appeal. Koster Keunen offers a comprehensive portfolio of high-quality waxes, all designed to meet the diverse needs of pharmaceutical and healthcare formulations.
In ingestible applications, waxes are utilized for tablet coatings to enhance swallowability, provide controlled drug release, and protect against environmental degradation. They also act as stabilizers in capsules and supplements, polishing agents, and non-stick coatings for gummy vitamins. In topical products, wax excipients improve texture, stability, and skin protection in formulations such as creams, balms, and gels, while enabling protective and sustained-release delivery systems.
Koster Keunen’s commitment to excellence in wax technology ensures that each excipient meets the rigorous demands of the pharmaceutical market. By offering unparalleled technical expertise, product consistency, and global supply capabilities, Koster Keunen supports formulators in developing innovative, effective, and reliable products. As a trusted partner in wax chemistries, Koster Keunen combines innovation and precision to empower the success of pharmaceutical solutions worldwide.
Wax Excipients for Ingestible Pharmaceuticals
Wax Excipients for Topical Pharmaceuticals
Wax Excipients For Pharmaceutical Applications
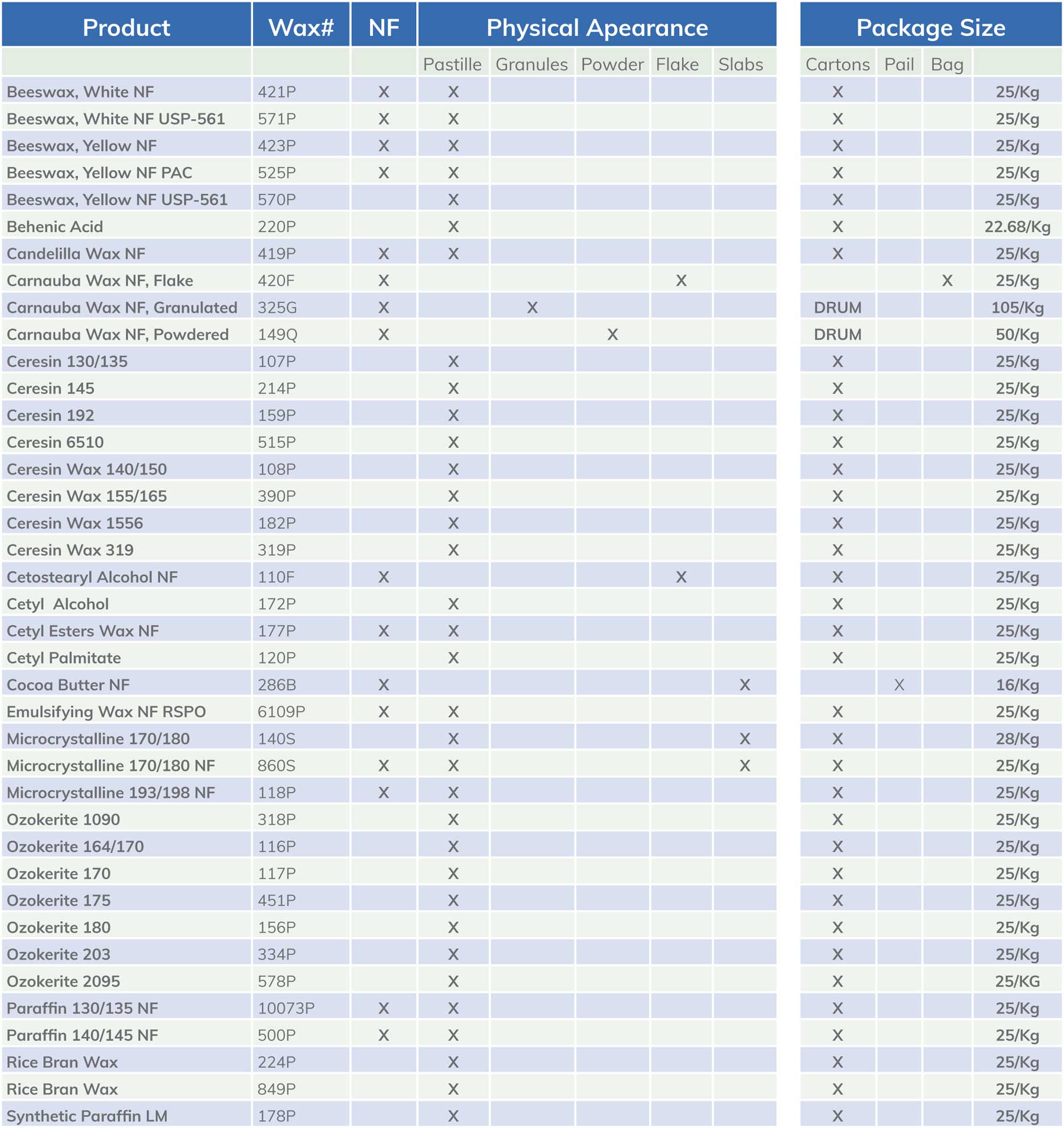
Beeswax
Beeswax is a natural, biocompatible excipient widely used in pharmaceutical formulations for both ingestible and topical applications. Derived from honeybee secretions, it provides structural integrity, controlled release properties, and emollient benefits. Recognized as Generally Recognized as Safe (GRAS) by the FDA and compliant with the United States Pharmacopeia (USP) standards, beeswax is a preferred ingredient in formulations requiring stability, extended release, and moisture retention. Its unique composition of esters, fatty acids, and hydrocarbons makes it an effective binding, emulsifying, and filmforming agent. Additionally, sustainably sourced beeswax aligns with the increasing demand for natural and eco-conscious pharmaceutical ingredients.
| PRODUCTS | |||
|---|---|---|---|
| Beeswax | White | NF | Wax #421P |
| Beeswax | White | NF USP-561 | Wax #571P |
| Beeswax | Yellow | NF | Wax #423P |
| Beeswax | Yellow | NF PAC | Wax #525P |
| Beeswax | Yellow | NF USP-561 | Wax #570P |
Applications
Ingestible
Topical & OTC Pharmaceuticals
Additional Information
Test Options Available
Microcrystalline Wax
Microcrystalline wax is a highly refined, versatile excipient widely used in pharmaceutical formulations for both oral and topical applications. Its fine crystalline structure, high molecular weight, and superior oil-binding capacity make it essential for controlled-release drug delivery, stabilization, and structural integrity. Recognized for its compatibility with a variety of polymers, resins, and oils, microcrystalline wax is particularly valuable in modified-release tablets, chewing gum-based drug delivery systems, and transdermal patches. Its barrier-forming properties also enhance product stability by protecting active ingredients from oxidation, moisture, and environmental degradation. With a long history of safe use, microcrystalline wax meets stringent pharmaceutical standards, ensuring efficacy and consistency in various dosage forms.
| PRODUCTS | ||
|---|---|---|
| Microcrystalline | 170/180 | Wax #140S |
| Microcrystalline | 170/180 NF | Wax #860S |
| Microcrystalline | 193/198 NF | Wax #118P |
Applications
Ingestible
Chewing Gum Drug Delivery:
Topical & OTC Pharmaceuticals
Additional Information

Paraffin Wax
Paraffin wax is a highly refined, hydrophobic excipient used in pharmaceutical formulations for its emollient, protective, and controlled-release properties. Its ability to create a moisture-resistant barrier makes it valuable in both topical and ingestible applications, improving drug stability, retention, and sustained release.
| PRODUCTS | ||
|---|---|---|
| Paraffin | 130/135 NF | Wax #10073P |
| Paraffin | 140/145 NF | Wax #500P |
Applications
Ingestible
Topical & OTC Pharmaceuticals

Carnauba Wax
Carnauba wax is a plant-derived excipient widely used in pharmaceutical coatings and controlled release formulations. Its high melting point, moisture resistance, and non-toxic nature make it valuable for enhancing tablet stability, improving swallowability, and modulating drug release.
| PRODUCTS | ||
|---|---|---|
| Carnauba Wax | NF Flake | Wax #420F |
| Carnauba Wax | NF Granulated | Wax #325G |
| Carnauba Wax | NF Powdered | Wax #149Q |
Applications
Ingestible
Topical & OTC Pharmaceuticals
Candelilla Wax
Candelilla wax is a plant-derived excipient valued for its hardness, binding properties, and ability to enhance stability in pharmaceutical formulations. It is commonly used in solid dosage forms for tablet binding, coating, and controlled-release applications.
| PRODUCTS | |
|---|---|
| Candelilla Wax NF | Wax #419P |
Applications
Ingestible
Medications Potentially Containing Candelilla Wax
Topical & OTC Pharmaceuticals

Topical & OTC Applications
The following waxes and fatty acids are widely used in topical and OTC pharmaceutical formulations due to their versatile properties, such as emulsion stabilization, thickening, and controlled release. These ingredients play a key role in enhancing the stability, texture, and performance of a wide range of creams, lotions, ointments, and other pharmaceutical products. Each compound is carefully selected for its ability to improve product efficacy and patient compliance, while also adhering to regulatory standards established by the FDA, USP, and NF.
Ozokerite Wax
Applications: Incorporated with oils to reduce greasiness and prevent sweating in formulations.
Ceresin Wax
Applications: Employed in protective, enteric, and sustained-release coatings for topical delivery systems.
Emulsifying Wax NF
Applications: A vegetable-based emulsifier used at approximately 5% to stabilize lotions, creams, and ointments.
Cetyl Palmitate
Applications: Found in moisturizers, gels, and ointments.
Cetylstearyl Alcohol
Applications: Functions as an emulsion stabilizer, opacifying agent, surfactant, and viscosity increasing agent.
Rice Bran Wax
Applications: Acts as a binder, thickener, and emulsifier; improves texture and stability of formulations.
Behenic Acid
Applications: Used in medications and ointments to enhance shelf life and efficacy.
Regulatory Compliance
All listed ingredients in this section are recognized in the United States Pharmacopeia (USP) and the National Formulary (NF) for pharmaceutical use.
The Food and Drug Administration (FDA) approves these substances for inclusion in topical and overthe-counter (OTC) pharmaceutical products.
Note: Ensure formulations comply with current USP, NF, and FDA guidelines.