Products
Formulas
The Benefits Of Using A Plasticizer In Personal Care
- Suppress Crystallization
- Creates Creamy Texture
- Supports Thermal Stability
- Inhibits Migration
- Enhances Dispersions
- Stops Bloom and Syneresis
Objectives
The objective of this article is to summarize the benefits of Kester Wax K-70P, a new plasticizer from Koster Keunen, Inc. In particular, the crystallization and rheological properties were investigated and correlated with the desired properties of an efficient plasticizer.
Introduction
Plasticizers are additives for increasing the flexibility and ease of processing. The presence of a plasticizer typically causes a reduction in the cohesive intermolecular forces along the wax molecules, enabling these chains to move more freely relative to one another, resulting in the reduction of stiffness of the wax matrix1.
Polyesters are among the commonly used plasticizers due to their favorable physical interactions with high molecular weight molecules that are typical constituents of waxes. This physical interaction between the wax and plasticizer molecules causes these materials to form a homogenous physical unit, meaning they do not separate.
The two main categories of plasticizers are primary and secondary. The former interacts with the wax molecules, while the latter increases the effectiveness of a primary plasticizer2. There are two types of plasticization with primary plasticizers: internal and external. Internal plasticization involves the chemical alteration of the wax molecule or its building blocks (prior to synthesizing the wax molecule). The second type of plasticization is external. External plasticization is the focus of this study. It is important to note that the plasticizer interacts with the wax physically, although hydrogen bonding and Van der Waals forces often play a role. Polyester plasticizers are favored across various industries and applications due to their exceptional flexibility.
Materials, Methods, and Instrumentation
Wax samples were prepared from Koster Keunen, Inc. commercial grade wax products with 5% w/w of plasticizer unless listed otherwise. Crystallization studies were carried out using an Olympus CH microscope using polarized light. Images were captured using a Moticam 3 3.0 MP camera integrated with the microscope. Microscope slides and cover glasses were purchased from Fisher Scientific and were electrically heated before cooling to ambient temperature for inspecting the crystallization process and the resulting crystalline particles.
Dynamic stress sweep rheology studies were conducted at the University of Connecticut, Institute of Materials Science Laboratory3. A Discovery HR20 rheometer (DHR20, TA) was employed utilizing a serrated Peltier plate fixture. The test configuration featured a modified cross-hatched 40 mm cone plate. Samples were loaded and zero gap determination was performed at the designated test temperature. A Peltier temperature control system connected to a recirculating water bath maintained the system at the required temperature throughout the tests. Yield stress measurements were performed using dynamic stress sweep experiments at 25 °C and 6.28 rad/s. The yield stress was determined from the cross over point (G′ = G′′)3.
Crystallization Behavior Of Natural Products
As previously stated, the crystallization process of natural waxes is influenced by factors such as temperature, cooling rate, and the presence of extraneous materials, e.g. impurities or additives. Typically, slow cooling results in the formation of larger, more well-defined crystals, whereas rapid cooling tends to produce smaller, less organized structures. Close attention to the crystallization behavior can enhance the performance and stability of products incorporating natural plant waxes.
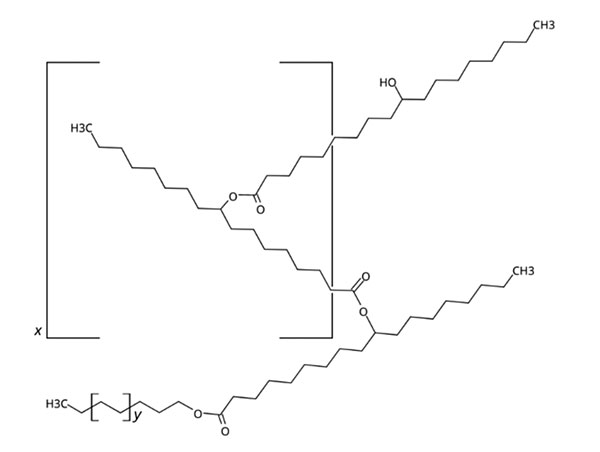
Figure 1. General chemical structure for Kester Wax K-70P
Plasticizing is accomplished by the plasticizer molecules embedding in the wax matrix between the crystalline molecules. Among the plasticizing theories, the free-volume theory explains that additional volume is created between the wax molecules (see Figure 1). The embedded plasticizer molecules will also provide more separation between the wax molecules weakening the interaction between them (gel theory) and providing lubricity (lubricity theory). These will modify the crystallization characteristics, alter the crystallization kinetics, and crystal morphology through reducing gel lattice network formation. The result is a more flexible and pliant wax matrix with a uniform distribution of smaller crystals throughout the materials. This reduces the materials’ brittleness and enhances the ability to withstand higher stress and deformation without fracturing, while maintaining the necessary protective and moisturizing properties. Understanding and controlling this crystallization behavior is essential for creating high-quality, effective personal care products that meet consumer expectations.
Crystallization Behavior Through PLM
Photo Groups 1-4, PLM Crystallization Photos at STP of natural waxes with and without Kester Wax K-70P.
This experiment employs polarized light microscopy (PLM) at standard temperature and pressure (STP) to examine the crystallization behavior of various natural waxes in the presence of Kester Wax K-70P. Eight PLM images, sanctioned into four photo groups, document the crystallization changes for each wax type. Each pair compares a control (10% wax with caprylic capric triglyceride (CCT) as a diluent to 100%) with a modified sample (15% wax where 5% of the CCT is replaced by Kester Wax K-70P).
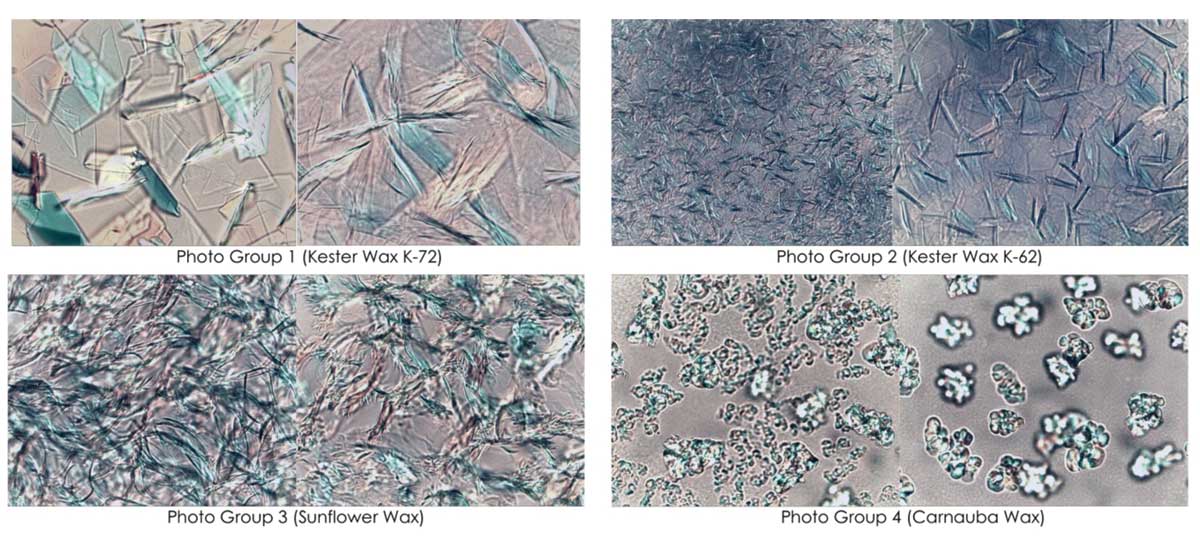
In Photo Group 1, the addition of Kester Wax K-70P into Kester Wax K-72 results in smaller crystals and reduced crystallization, with noticeable interference in platelet crystal formation leading to finer crystals.
In Photo Group 2, the addition of Kester Wax K-70P into Kester Wax K-62 reduces crystallization and improves the amorphous gel-like structure.
In Photo Group 3, the addition of Kester Wax K-70P into Sunflower Wax exhibits a reduction in both crystal size and crystal count.
In Photo Group 4, the addition of Kester Wax K-70P into Carnauba Wax decreases crystal formation and promotes a more amorphous appearance in the sample.
These results suggest Kester Wax K-70P significantly alters crystallization behaviors across different waxes, favoring reduced crystal formation and increased amorphous characteristics.
Flow Properties With Kester Wax K-70P
Rheology, being the study of flow and deformation properties of a wide range materials, is a convenient and valuable tool for determining the effect of plasticizers on these physical properties of wax matrices. Dynamic stress sweep tests were performed on an All Over Body Balm (refer to Formula 2) containing both no plasticizer and 15% Kester Wax K-70P.
At low stress amplitudes, both the elastic G′ and the viscous G′′ modulus formed a constant plateau region known as the linear viscoelastic range (LVR) of the materials. In the LVR, the stress induced in response to imposed strain occurs in proportionality and, therefore, a linear range is found. For all studied body balms, G′ was observed to be higher than G′′ in the LVR, revealing a dominating elastic character of the products at low stress range (refer to Figures 2 and 3). The greater the elastic nature of the products leads to a substantial elongation of the structural network under continually increasing straining/stress force before the structure is distorted into a state of viscoelastic liquid.
| Formula 2 Base Formula (No Plasticizer) | Formula 2 Base Formula +15% Kester Wax K-70P | ||
|---|---|---|---|
| Modulus (Pa) | Stress (Pa) | Modulus (Pa) | Stress (Pa) |
| 155.5 | 45581.9 | 131.9 | 44544.5 |
Table 1. Modulus and Stress values for All Over Body Balm in absence and presence of Kester Wax K-70P
Inclusion of a plasticizer within a formulation, ideally by substituting a portion of the oil phase, can provide substantial benefits to the overall product matrix. By replacing a portion of the oil rather than interacting with the wax or structurant, the plasticizer functions to modify the crystalline properties subtly without compromising the structural components. This targeted approach not only reduces crystallization but also enhances thermal stability and supports the cohesive integrity of the formulation. The role of plasticizing waxes in this context is instrumental in stabilizing the product, ultimately contributing to improved durability and performance across various conditions.
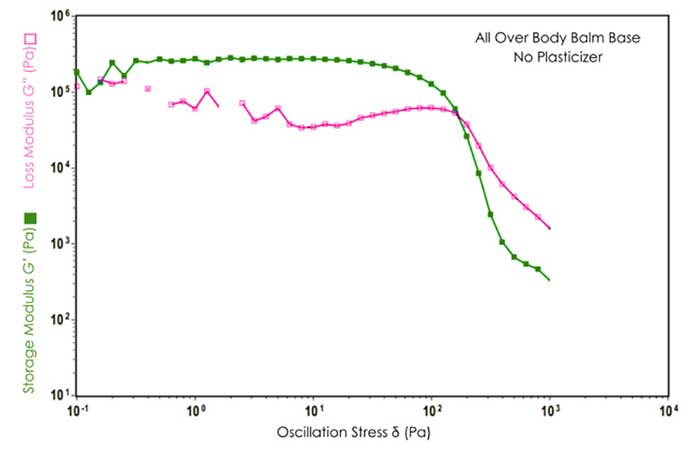
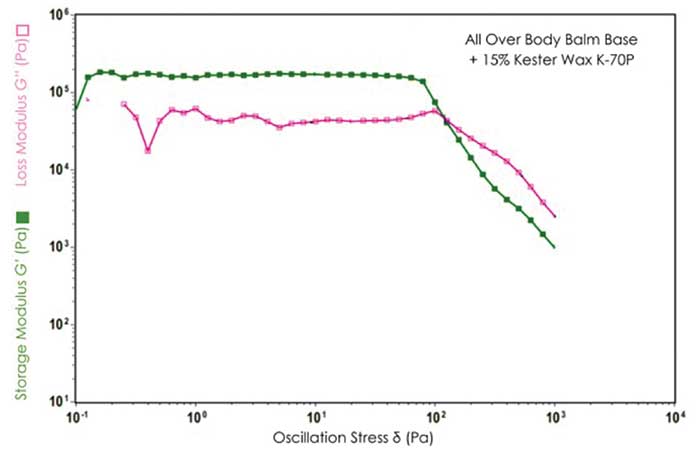
Figure 2 & Figure 3. Dynamic Stress Sweep results for All Over Body Balm in absence and presence of Kester Wax K-70P.
Formulation Consistency Optimization
In developing formulations with various structuring wax-to-plasticizer ratios, careful adjustment of processing and pour temperatures is critical to achieving the desired consistency and structure. The ideal approach is to pour formulations at temperatures 7–10°C above the specific congeal point, allowing the structured components to solidify into a stable matrix. Conversely, pouring or processing below the congeal point down to room temperature yields a softer product texture. Note that pouring at the congeal point can negatively impact the formula’s structural integrity and should be avoided. This precise control of temperature in relation to the congeal point across formulations with different structurant levels enables formulators to balance structural integrity with flexibility, tailoring product hardness and stability to meet diverse functional needs (see Figure 4).
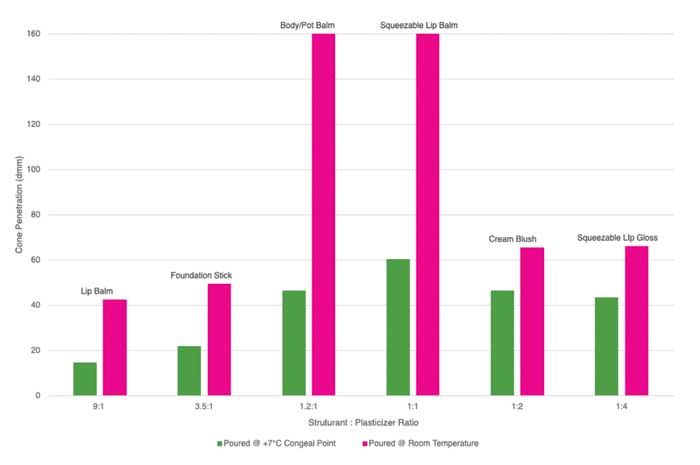
Figure 4. The cone penetration numbers measured with varying Structurant: Plasticizer ratios of common anhydrous formulas pouring 7°C above congealing point and at room temperature.
Formulas
Squeezable Lip Balm
| Ingredient Trade Name | INCI Name | % |
|---|---|---|
| Phase A | ||
| Sunflower Wax | Helianthus Annuus (Sunflower) Seed Wax | 5.0 |
| Kester Wax 70P | C18-38 Alkyl Hydroxystearoyl Stearate | 5.0 |
| Coconut Oil | Cocos Nucifera (Coconut) Oil | 30.0 |
| Jojoba Oil | Simmondsia Chinensis (Jojoba) Seed Oil | 30.0 |
| Jeechem CTG | Tocopheryl Acetate | 30.0 |
All Over Body Balm
| Ingredient Trade Name | INCI Name | % |
|---|---|---|
| Phase A | ||
| AR Cocoa Butter | Theobroma Cacao (Cocoa) Butter | 12.0 |
| Sunflower Wax | Helianthus Annuus (Sunflower) Seed Wax | 7.5 |
| Plasticizer* | 15.0 | |
| Kester Wax K-24 | Lauryl Laurate | 8.0 |
| Coconut Oil | Cocos Nucifera (Coconut) Oil | 34.0 |
| Jojoba Oil | Simmondsia Chinensis (Jojoba) Seed Oil | 13.5 |
| Rice Bran Oil | Oryza Sativa (Rice Bran) Oil | 9.0 |
| Vitamin E Acetate | Tocopheryl Acetate | 1.0 |
*Plasticizer used: Kester Wax K-60P (Bar structure; Pen=35.5), Kester Wax K-70P (Twist-up, Pen=46), Kester Wax K-82P (Soft pot, Pen=107).
Conclusion
In line with the objectives of this article we have investigated the efficiency of Kester Wax K-70P for changing the morphological and rheological properties of certain wax matrices. These properties and modification of these properties are crucial for a plasticizer. The experimental results support the requirements described in the scientific literature. By modifying the crystallization and flow/ deformation properties of the wax matrices subjected to this research, Kester Wax K-70P, our assessment is that this material is a promising addition to the plasticizer market. Since it is globally accepted and made from naturally sourced ingredients, it will greatly benefit the users, formulator in the personal care industry where improved workability and quality is desired.
References
- Wypych, G. Handbook of Plasticizers (Fourth Edition), ChemTec Publishing, 2023.
- Raloff, J. Science News. Sept 9, 2000, v158 ill p165.
- University of Connecticut Institute of Materials Science Industrial Affiliates Program.

